
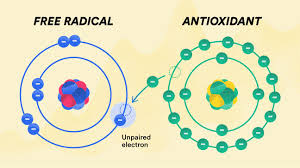
Free radicals are highly reactive molecules or atoms that contain one or more unpaired electrons. Because of their unstable nature, they interact with other molecules in the body, often damaging cells, proteins, and DNA. Free radicals play a role in aging and various diseases, but they also have important biological functions.
Relevant keyword details
1. Reactive Oxygen Species (ROS)
- Superoxide (O₂⁻)
- Hydroxyl Radical (OH·)
- Hydrogen Peroxide (H₂O₂)
- Vitamin C
- Vitamin E
- Glutathione
6. Mitochondria
7. Free radical scavengers
8. DNA damage
Types of Free Radicals
Free radicals can be classified based on their chemical structure, origin and reactivity. Here are the main types of free radicals:
1. Reactive Oxygen Species (ROS)
Reactive Oxygen Species (ROS) are the most common type of free radicals and are derived from oxygen. These include:
- Superoxide Anion (O₂⁻): When oxygen gains an extra electron, it is highly reactive but can be converted into more harmful species.
- Hydroxyl radical (OH·): one of the most reactive free radicals, generated from hydrogen peroxide (H₂O₂) and involved in lipid peroxidation and DNA damage.
- Hydrogen Peroxide (H₂O₂): Although not a free radical itself, it is considered a ROS because it can generate hydroxyl radicals.
- Singlet oxygen (¹O₂): A highly reactive excited form of oxygen, often involved in reactions with lipids and proteins.
- Peroxyl radical (ROO·): Formed during oxidation of lipids, which damages cell membranes.
- Alkoxyl radical (RO·): Like peroxyl radicals, these are involved in lipid peroxidation and are generated during the breakdown of peroxides.
2. Reactive Nitrogen Species (RNS)
Reactive Nitrogen Species (RNS) are free radicals derived from nitrogen and are also highly reactive.
Nitric oxide (NO·): A signaling molecule that is involved in many physiological processes but can become harmful when combined with superoxide to form peroxynitrite.
Peroxynitrite (ONOO⁻): Formed by the reaction of nitric oxide and superoxide, this is a highly reactive species that can damage proteins, lipids, and DNA.
Nitrogen dioxide (NO₂·): Another reactive nitrogen species that can cause oxidative damage, particularly in the lungs.
3. Carbon-centered radicals
These radicals contain carbon and are often the result of metabolic processes.
Alkyl radicals (R·): Formed during the breakdown of organic compounds, these radicals are involved in organic reactions and can contribute to lipid peroxidation.
Methyl radical (CH₃·): A type of alkyl radical that is produced in organic reactions and reacts with oxygen to form ROS.
4. Lipid radicals
They are produced during the peroxidation of lipids in cell membranes.
Lipid peroxyl radicals (LOO·): generated during lipid oxidation, particularly affecting polyunsaturated fatty acids in cell membranes.
Lipid Alkoxyl Radicals (LO·): These are intermediates in lipid peroxidation chain reactions, which cause membrane damage.
5. Thiel Radicals (RS·)
These are sulfur-centered radicals, often formed from thiol (sulfur-containing) compounds such as cysteine or glutathione.
Thiel radical (RS·): involved in the oxidation and modification of proteins, causing cellular damage.
6. Other types of free radicals
Halogen radicals: These include chlorine (Cl·), bromine (Br·), and iodine (I·) radicals, which are involved in various chemical reactions, including atmospheric reactions that can affect ozone depletion.
Transition Metal Radicals: Metals such as iron (Fe²⁺, Fe³⁺) and copper (Cu⁺, Cu²⁺) can participate in free radical generation through the Fenton reaction, which produces hydroxyl radicals from hydrogen peroxide.
These radicals play important roles in biological processes such as signaling, immune responses, and cellular respiration, but when present in high or uncontrolled amounts, they can also contribute to disease.
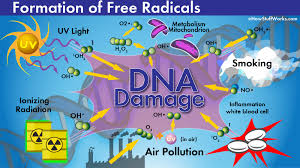
what causes free radicals in the body?
Free radicals in the body can be generated by a number of internal and external factors. The production of free radicals is a natural part of cellular processes, but exposure to certain conditions can significantly increase their levels, leading to oxidative stress.
Here is an overview of the main reasons:
1. Cellular metabolism (endogenous sources)
Free radicals are byproducts of normal cellular processes, particularly during energy production in the mitochondria.
Mitochondrial Respiration: As cells produce energy (ATP) through oxidative phosphorylation, some electrons are released from the electron transport chain and react with oxygen, producing reactive oxygen species such as superoxide (O₂⁻). ROS) is formed.
Enzymatic reactions: Certain enzymes, such as NADPH oxidase and xanthine oxidase, generate free radicals as part of cellular signaling or immune defense.
2. Inflammation
The body’s immune system produces free radicals as part of its defense against infection.
White blood cells (phagocytes): During an immune response, phagocytes (eg, neutrophils and macrophages) produce free radicals such as superoxide and nitric oxide to destroy pathogens.
Chronic inflammation: Long-term inflammatory conditions (eg, arthritis, asthma) can lead to overproduction of free radicals, which cause tissue damage.
3. Exposure to Environmental Factors (External Sources)
Many external factors can increase the production of free radicals in the body, including:
Pollution: Air pollutants such as ozone, nitrogen dioxide, and particulate matter increase the production of free radicals when inhaled, which contribute to oxidative stress in the lungs and other tissues.
Tobacco smoke: Cigarette smoke contains a large number of free radicals and chemicals that promote the formation of ROS, which cause oxidative damage to the lungs, skin, and cardiovascular system.
Radiation (UV and Ionizing): Ultraviolet (UV) light from the sun and ionizing radiation (eg, X-rays, gamma rays) can break chemical bonds in skin cells and cause free radical formation. This causes DNA damage and aging of the skin.
Pesticides and industrial chemicals: Exposure to toxic chemicals, such as those found in pesticides, can trigger free radical production, which can damage organs over time.
4. Diet and lifestyle factors
Certain dietary choices and lifestyle habits can increase the level of free radicals in the body:
Unhealthy diet: Processed foods, trans fats and sugary foods can stimulate the production of free radicals. Cooking methods such as frying, grilling or barbecuing can also create free radicals in food.
Alcohol consumption: Excessive alcohol consumption leads to the formation of free radicals, especially in the liver, as it is metabolized.
Physical exercise: While moderate exercise boosts antioxidant defenses, intense or prolonged physical activity can lead to overproduction of free radicals due to increased oxygen consumption.

5. Heavy metals and transition metals
Exposure to heavy metals such as lead, mercury, cadmium, and transition metals such as iron and copper can increase the formation of free radicals.
Fenton reaction: Transition metals (eg, iron and copper) can catalyze the formation of highly reactive hydroxyl radicals (OH·) from hydrogen peroxide (H₂O₂), which contribute to cellular damage. are
6. Stress and emotional factors
Psychological stress is associated with increased oxidative stress due to the release of stress hormones such as cortisol and adrenaline.
These hormones can alter metabolism, leading to overproduction of free radicals.
7. Drugs and medicines
Some drugs, such as chemotherapy agents, can increase free radical production as part of their mechanism of action or as a side effect. For example:
Chemotherapy: Many cancer treatments create free radicals to damage the DNA of cancer cells, but can also affect healthy cells.
Acetaminophen overdose: An overdose of acetaminophen (paracetamol) can damage the liver through the formation of free radicals as the drug is metabolized.
8. Old age
As we age, the body’s antioxidant defenses weaken, and mitochondrial function declines, leading to increased free radical production and increased risk of oxidative damage to tissues and organs.
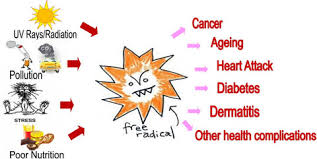
Summary
Free radicals are produced naturally in the body, but their levels can increase because:
- Intrinsic factors such as metabolism, inflammation and aging.
- External factors such as pollution, smoking, UV exposure, diet, alcohol, stress and certain medications.
- An imbalance between free radicals and antioxidants leads to oxidative stress, which is associated with various diseases such as cancer, cardiovascular disease, neurodegenerative disorders, and aging.






